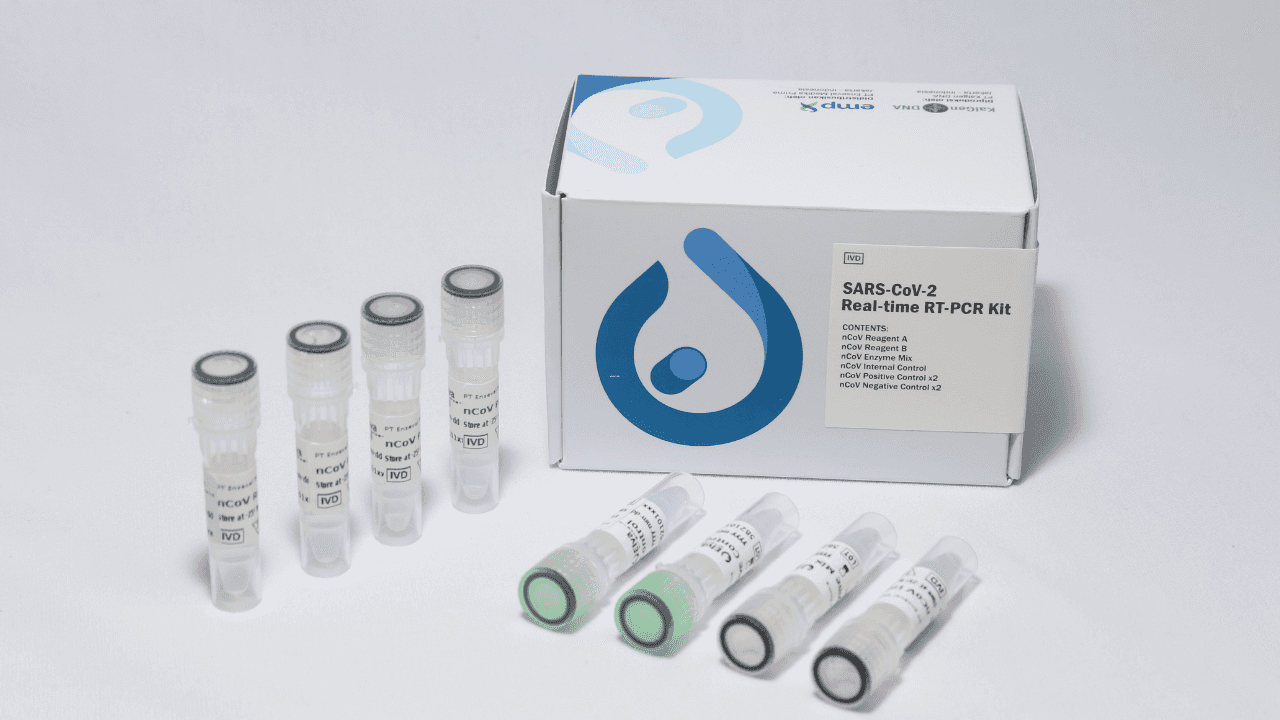
Elva Diagnostic SARS-CoV-2 Real-time RT-PCR Kit is a real-time RT-PCR in vitro diagnostic test intended for the qualitative detection of nucleic acid from the SARS-CoV-2 virus in human oropharyngeal swab and nasopharyngeal swab specimens .
About Us
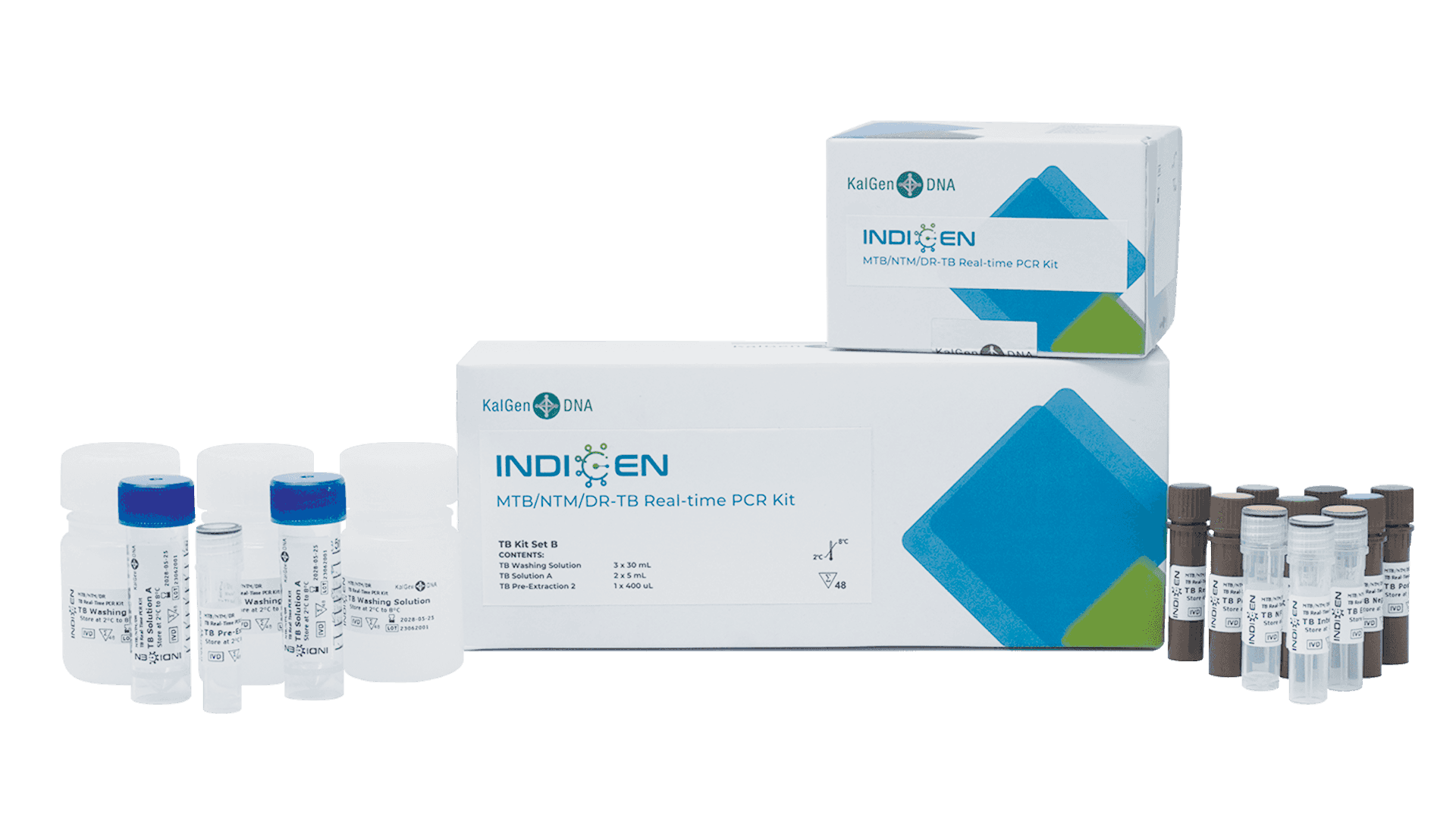
PT. KalGen DNA was established in 2012, is a joint venture between PT Bifarma Adiluhung (a subsidiary of PT Kalbe Farma Tbk.) and a Malaysian company, DNA Laboratories Sdn. Bhd., which is a leading provider of genetic screening and molecular diagnostic services and products in Malaysia.
Our commitment to quality and improvement are demostrated by well-trained molecular analyst, certification by WHO LabNet proficiency test, ISO 13485, obtained CPAKB, CDAKB permit from Ministry of Health. We will continue to develop innovative diagnostic kits in the area of personalized healthcare to improve human health and well-being.
Read More

We are Certified Manufacturing Facility to Ensure Quality & Safety
Mission
To Improve Human Health and Well Being through Innovative Diagnostic Kits.
Vision
To be The Most Reliable Partner of High Quality Diagnostic Kits, driven by Innovation and Excellent Management
Our Services
A well-proven way to prevent cervical cancer is to have screening test. Cervical cancer can affect anyone with a cervix who has ever been sexually active.
KalGen DNA as a marketing for cervical cancer screening test, cooperate with collaborator laboratory, encourage anyone with a cervix who has been sexually active to be screened for cervical cancer.
The fact that cervical cancer rarely presents any symptoms in its early stages highlights the importance of regular screening for the disease.
The main goal of cervical screening is to detect and treat precancer before cancer develops.